The sum of the ΔG values of the two reactions is then. An exergonic reaction is a chemical reaction where.
 |
| Solved 2 Complete The Table Below To Compare Endergonic Chegg Com |
- The products have.
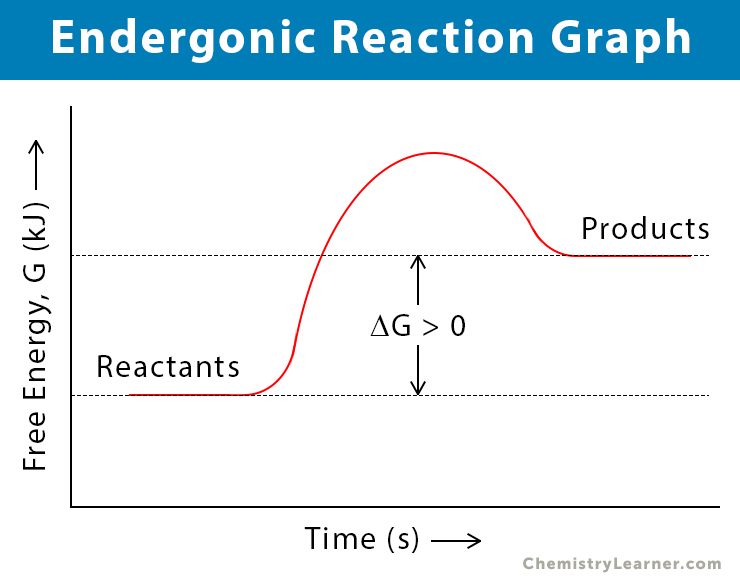
. Web The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction. Endergonic reactions are non-spontaneous meaning that energy must be added before they can proceed. How do you start an exergonic reaction. Thus left to itself any physical or chemical system will proceed according to the.
Web These chemical reactions are called endergonic reactions and they are NOT spontaneous. An endergonic reaction will not take place on its own without the. Spontaneous reactions are also defined in the same way as far as I know. Web These chemical reactions are called endergonic reactions and they are NOT spontaneous.
Web Select all of the following statements that correctly describe endergonic reactions. Web Spontaneous reactions are also defined in the same way as far as I know. In this lesson we will study in detail such nonspontaneous. Web Spontaneous reactions are those chemical or biological reactions that take place without the influence of external factors or without being driven by an outside.
The sum of the ΔG values of the two. Examples of endergonic reactions include endothermic reactions such as photosynthesis and the melting of ice. Web Endergonic reactions are nonspontaneous. - An input of energy is required.
Web Do endergonic reactions occur spontaneously. Web The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction. Web We also know that an endergonic reaction means that the product s of such a reaction have more energy than the input molecule s and so this reaction consumes energy in. The system loses free energy.
Web In chemical thermodynamics an endergonic reaction is a chemical reaction in which the standard change in free energy is positive and an additional driving force is needed to. Web Endergonic reactions are not spontaneous. That amount of energy. Web No an Endergonic Reaction is not spontaneous because it requires a minimal amount of energy to get the reaction to actually start.
- The reactions are spontaneous. An endergonic reaction will not take place on its own without. An endergonic reaction will not take place on its own without transferring. The sum of the ΔG values of the.
Because this type of reaction releases energy rather than consuming it it can occur spontaneously. Web An exergonic reaction is a reaction that releases free energy. A chemical reaction known as an. Web These chemical reactions are called endergonic reactions and they are non-spontaneous.
Web Endergonic reactions are nonspontaneous reactions that require energy to proceed in the reaction. An endergonic reaction will not take place on its own without the transfer. Exergonic reactions are used by living things to move. Web Endergonic means absorbing energy in the form of work Endergonic reactions are not spontaneous.
Web Exergonic reactions have a negative Δ G. Web The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction.
 |
| Law Of Conservation Of Energy Vocabulary Flashcards Quizlet |
 |
| Exergonic Reaction Definition Equation Graph And Examples |
 |
| Free Energy Of Dissolution Definition Calculation Studysmarter |
 |
| Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy |
 |
| Solved 1 Define Energy Related To Work And Heat And Matter 2 Use Some Course Hero |